Samples
A total of 31 cremated bone samples from 25 individuals were analyzed, all of which have been previously radiocarbon dated
3. Of the 25 individuals, 24 came from the excavation of the Aubrey Hole 7 where they had been re-buried in 1935. The last individual, a woman, was found just next to Aubrey Hole 7. Three samples represent juveniles while the remainder are adults. While it is difficult to sex cremated remains, it was still possible to identify six possible females and nine possible males, with the remainder unsexed
3. In addition, 17 modern plant samples from west Wales were analysed to better characterize the biologically available strontium around the quarries where bluestones originate.
Infrared analyses
The cremated bone samples were first crushed using a mortar and pestle and then analysed by FTIR-ATR (Agilent Technologies Cary 640 FTIR with GladiATR
TM from Pike Technologies). Each sample was pressed onto the diamond crystal and measured in triplicate (on different aliquots if sufficient material was available). The background was measured and removed and a baseline correction was carried out using Agilent Resolution Pro. The spectra were normalised and averaged before calculation of a suite of infrared indices that describe the composition and structure of the bone
10.
Various infrared indices were calculated for each sample (Table
S1 – see 8, 10 for more details). The cyanamide to phosphate ratio (CN/P) is of particular interest as it characterizes the presence of cyanamide, which has been suggested to be present when bone is burned in reducing conditions
10,11. The IRSF has been shown to be temperature dependent while the BPI characterizes the amount of remaining carbonates present in bone after cremation.
Carbon and oxygen stable isotope ratios
Before isotopic analysis, the calcined bone fragments were treated with sodium hypochlorite (1% NaOCl) for one hour to remove any remaining organic matter followed by three rinses with MilliQ water and then a four-hour treatment with 1 M acetic acid (CH3COOH) to remove any adsorbed carbonates and diagenetic apatites. The samples were then rinsed three times with MilliQ water and left in the freeze-dryer overnight. Between 3 and 5 mg were reacted with 100% phosphoric acid at 70 °C for 4 min in a Kiel IV autocarbonate device interfaced with a Delta V Advantage isotope ratio mass spectrometer at the SSMIM (Service de Spectrométrie de Masse Isotopique du Muséum National d’Histoire Naturelle), Paris, France. A laboratory carbonate standard (LM marble) normalized with NBS 19 and giving mean δ13C and δ18O values of +2.08 ± 0.04‰ and +1.70 ± 0.05‰, respectively, (n = 78) was used to check the accuracy and precision of the data. We used these standard deviations as an indicator of analytical precision over the period of analysis. The carbon and oxygen isotope ratios are, by convention, expressed relative to a standard (VPDB – Vienna Pee Dee Belemnite). Both are expressed in per mil (‰ – 1/1000):
δ13C=(C13/C12)sample−(C13/C12)vPDB(C13/C12)vPDB×1000&δ18O=(O18/O16)sample−(O18/O16)vPDB(O18/O16)vPDB×1000
Strontium isotope analysis
Two isotopes of strontium,
86Sr and
87Sr, are widely used in mobility studies of humans and fauna. Strontium-87 is the product of the radioactive decay of rubidium-87 (
87Rb), so strontium isotope ratios (
87Sr/
86Sr) vary between different types of bedrock, depending on the initial
87Sr/
86Sr ratio, the Rb/Sr ratio and the age. The older and more Rb-enriched the bedrock, the more enriched it is in
87Sr
31. Soluble strontium is then taken up by plants and enters the bones and teeth of humans and animals by replacing calcium in the bioapatite fraction of bone and teeth. Hence, strontium isotope ratios (
87Sr/
86Sr) can be measured on bone and teeth to suggest places of origin for animals and humans. The residence time of strontium in bone apatite is not fully known but here we assume that it will be around 10 years.
Strontium isotope ratios were measured by Multi-Collector Inductively-Coupled-Plasma Mass-Spectrometry (MC-ICP-MS) following the procedure detailed in
12. Cremated bones were pretreated with 1 M acetic acid (1 mL per 10 mg of sample) for 3 min in an ultrasonic bath, followed by three rinses with MilliQ water and 10 min ultrasonication. Plant samples were simply ashed in a muffle furnace at 650 °C. The entire acid digestion process and subsequent Sr purification were achieved under a class 100 laminar flow hood in a class 1000 clean room (Université Libre de Bruxelles, Belgium, hereafter ULB). Fifty mg of sample were digested in subboiled concentrated HNO
3 at 120 °C for 24 h, before purification of the Sr analyte by a chromatographic technique on ion-exchange resins (see 12 for more details). The isotope ratios of the purified strontium samples were then measured on a Nu Plasma MC-ICP mass spectrometer (from Nu Instruments) at ULB. During the course of this study, repeated measurements of the NBS987 standard solution yielded
87Sr/
86Sr = 0.710214 ± 40 (2 SD for 15 analyses), which is, for our purposes, largely sufficiently consistent with the average value of 0.710252 ± 13 (n = 88) obtained by TIMS (Thermal Ionization Mass Spectrometry)
32. All the data were corrected for mass fractionation by internal normalization to
86Sr/
88Sr = 0.1194. In addition, after the measurements all the rough data were normalised using a standard-sample bracketing method with the recommended value of
87Sr/
86Sr = 0.710248
32. For each sample the
87Sr/
86Sr value is reported with a 2σ error (absolute error value of the individual sample analysis − internal error).
Strontium concentrations
Small sample fractions (~1 to 3 mg) pre-treated as above were digested in precleaned Teflon beakers (Savillex) using subboiled 7 M HNO
3 at 120 °C for 24 h, evaporated to near-dryness and subsequently digested with a drop of concentrated HNO
3. Following dilution with 2% HNO
3, Sr and Ca concentrations (ppm) in the sample digests were determined using a Thermo Scientific Element 2 sector field ICP mass spectrometer at the Vrije Universiteit Brussel (VUB, Belgium) in low (
86Sr and
88Sr) and medium (
43Ca and
44Ca) resolution using Indium (In) as an internal standard and external calibration versus a calibration curve. Accuracy was evaluated by the simultaneous analysis of limestone reference material SRM8544 (NBS19) and comparison to available published literature data
33,34. Based on repeated digestion and measurement of this reference material, the analytical precision (1 SD) of the procedure outlined above is estimated to be better than 3%.
Statistical analysis
Data were assessed for normality using Shapiro-Wilk tests and then analysed with parametric (Student’s t) or non-parametric (Mann-Whitney U) tests as appropriate. For distributions not departing significantly from normality, Cohen’s
d was used to assess effect size, a measure of the strength of a difference between two sample means, beyond its simple existence. By convention,
d values of less than 0.2 (the difference in sample means expressed in pooled standard deviations) are taken to be small (i.e., meaningless regardless of the
p value), while those above 0.7 are considered large
35. For non-normal distributions, the strength of the relationship between the two variables was assessed using Spearman’s correlation coefficient
rho36.
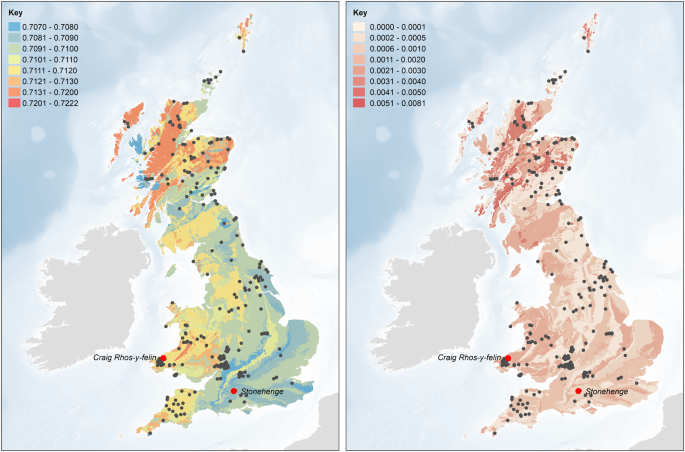
Map of the biologically available strontium
An updated version of the map of biosphere strontium isotope variation across Britain (Fig. 1b in
14) was generated using British Geological Survey 1:625,000 bedrock geology digital mapping (DiGMapGB-625 – Figure
S1) and additional strontium data from key regions in southern Britain. The dataset from Evans
et al. 2010 was supplemented with data for plants growing on key rock types in south-west Wales (Table
S2), the Gower peninsula (Schulting
et al. n.d.), Gloucester
19 and the Ridgeway
15. Mean values and standard deviations were calculated for samples corresponding to each polygon in the DiGMapGB-625 dataset. Where possible, only values from plant samples were used to calculate the mean values and standard deviations for each polygon. Where no plant data were available, mean values and standard deviations were calculated, in order of preference, as follows:
- (1)
Other samples – using values from bone, dentine, soil and water samples;
- (2)
Rock types – using values from other polygons of the same rock type;
- (3)
Isotope packages – using values for other polygons of the same isotope package.
Outliers, identified as those measurements lying more than 3 IQR (interquartile range) in their isotope package, were removed from the analysis (Figures
S2 and
S3). The bedrock polygons were converted to a raster dataset with a cell size of 100 m (to ensure that small polygons were retained, and cell values corresponding to the mean value of the dominant rock type (the rock type with the largest combined area)). A baseline of biologically available strontium was created by calculating the focal mean of each cell in the raster dataset, i.e. the mean value of all cells within a 5 km search radius, using an extension of the methodology employed for the analysis of strontium data from Neolithic and Bronze Age sites in Northern Ireland
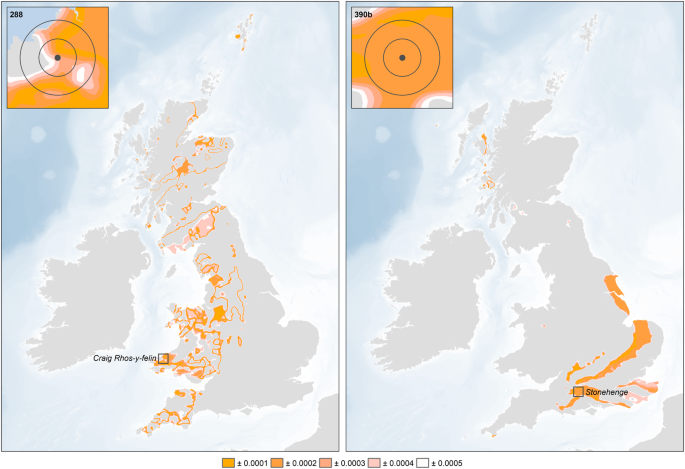
17. Continuous surfaces showing the absolute deviation from the focal mean were subsequently generated for each of the individuals from Stonehenge, with cells with the lowest values representing locations with focal means that are similar to the measured values, i.e. the locations from which the individuals are most likely to have originated (Figures
S4,
S5and
S6).
Defining ‘local’
In the absence of wheeled transport, small-scale, intensive agriculture being proposed for the Neolithic and Bronze Age tends to focus on fields within 1 km of settlements
37,38. Of course use would also be made of more distant fields, but since the Cretaceous chalk extends for at last 15 km in all directions around Stonehenge its strontium isotope range should provide a robust definition of ‘locals’. Those individuals making repeated use of fields with significantly higher
87Sr/
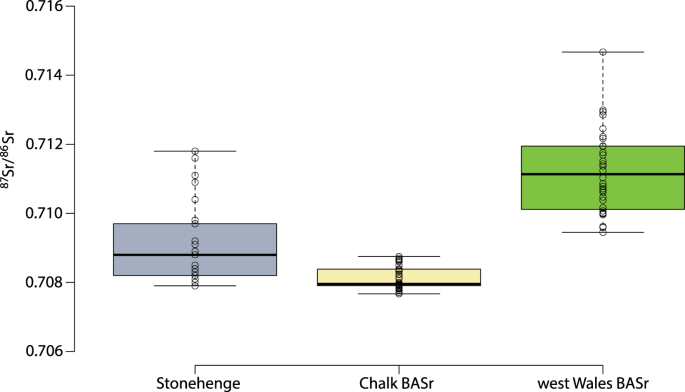
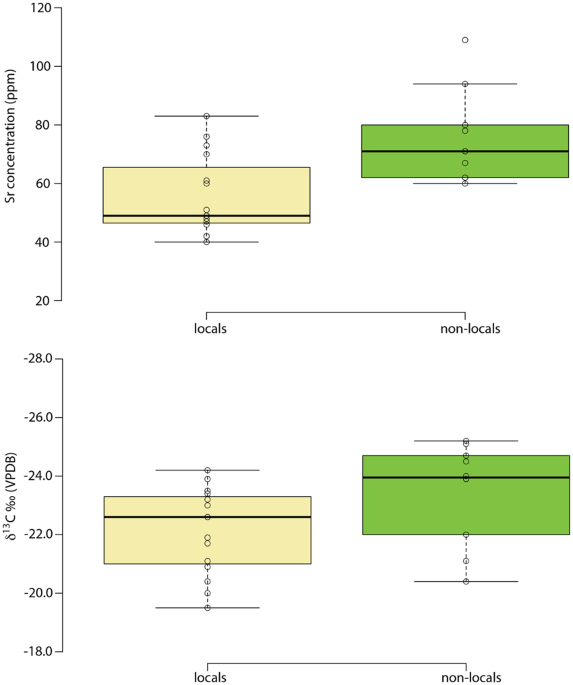
86Sr values to the west or north would be expected to exhibit higher values in their bone, which presents an average of bioavailable strontium in foods consumed over the last decade or so of life. Given the mobility of cattle and sheep, some pastures may also have been at greater distances from settlements, but meat and milk contain relatively little strontium compared to plants, and so the contribution made by livestock would be heavily biased against. As such, using the updated version of the map of the biologically available strontium for the UK it is possible to define ‘local’ and ‘non-local’ individuals. ‘Locals’ are characterized by values below 0.7090 (Fig.
4). The origin of the ‘non-locals’ was evaluated using the continuous surfaces created for each individual (see above and Figs
3,
S4,
S5 and
S6).